Agates from Western Atlas (Morocco)—Constraints from Mineralogical and Microtextural Characteristics
Abstract
:1. Introduction
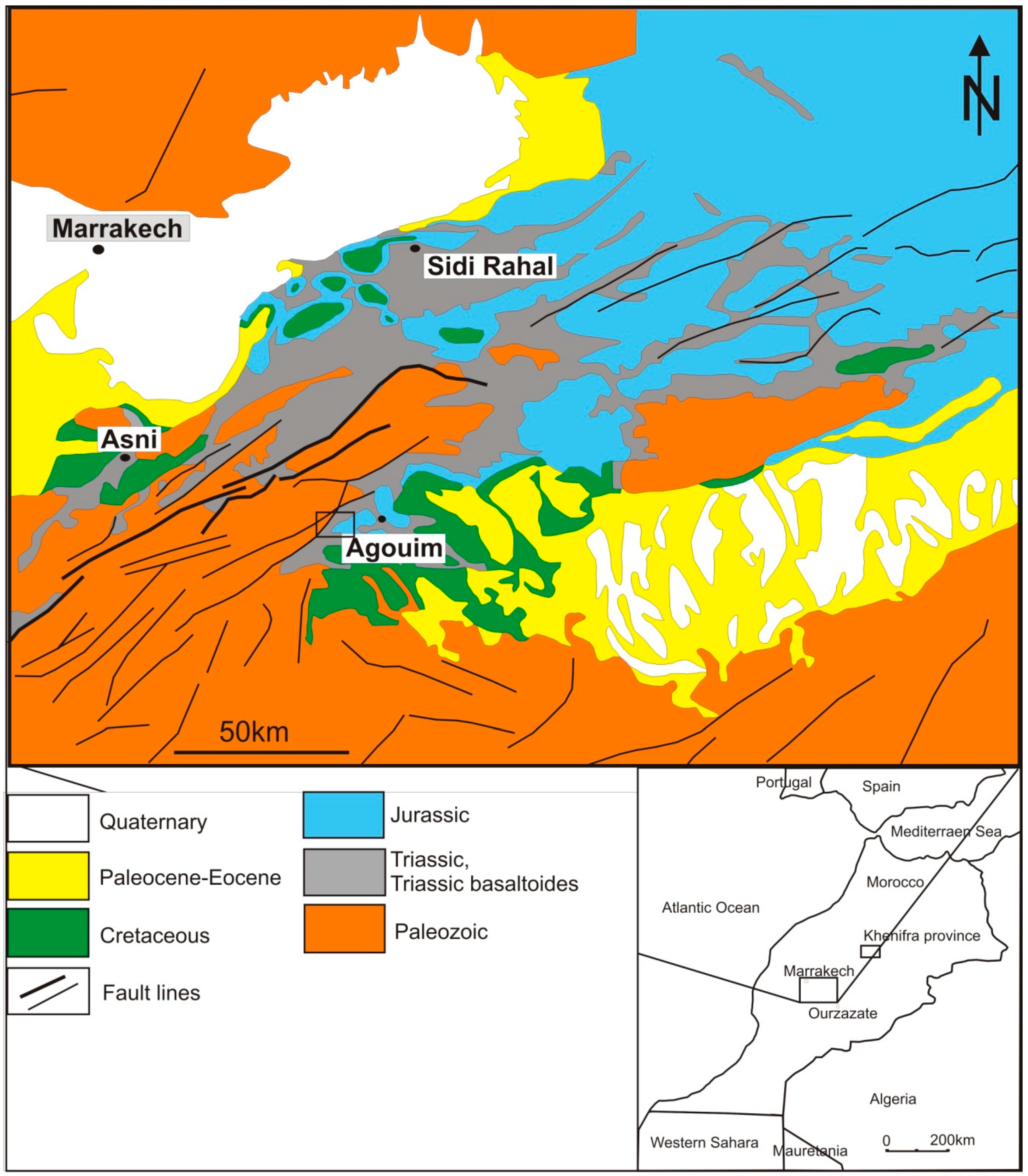
2. Geological Settings and Localizations of Agates Occurrences in Morocco
3. Material and Methods
4. Results
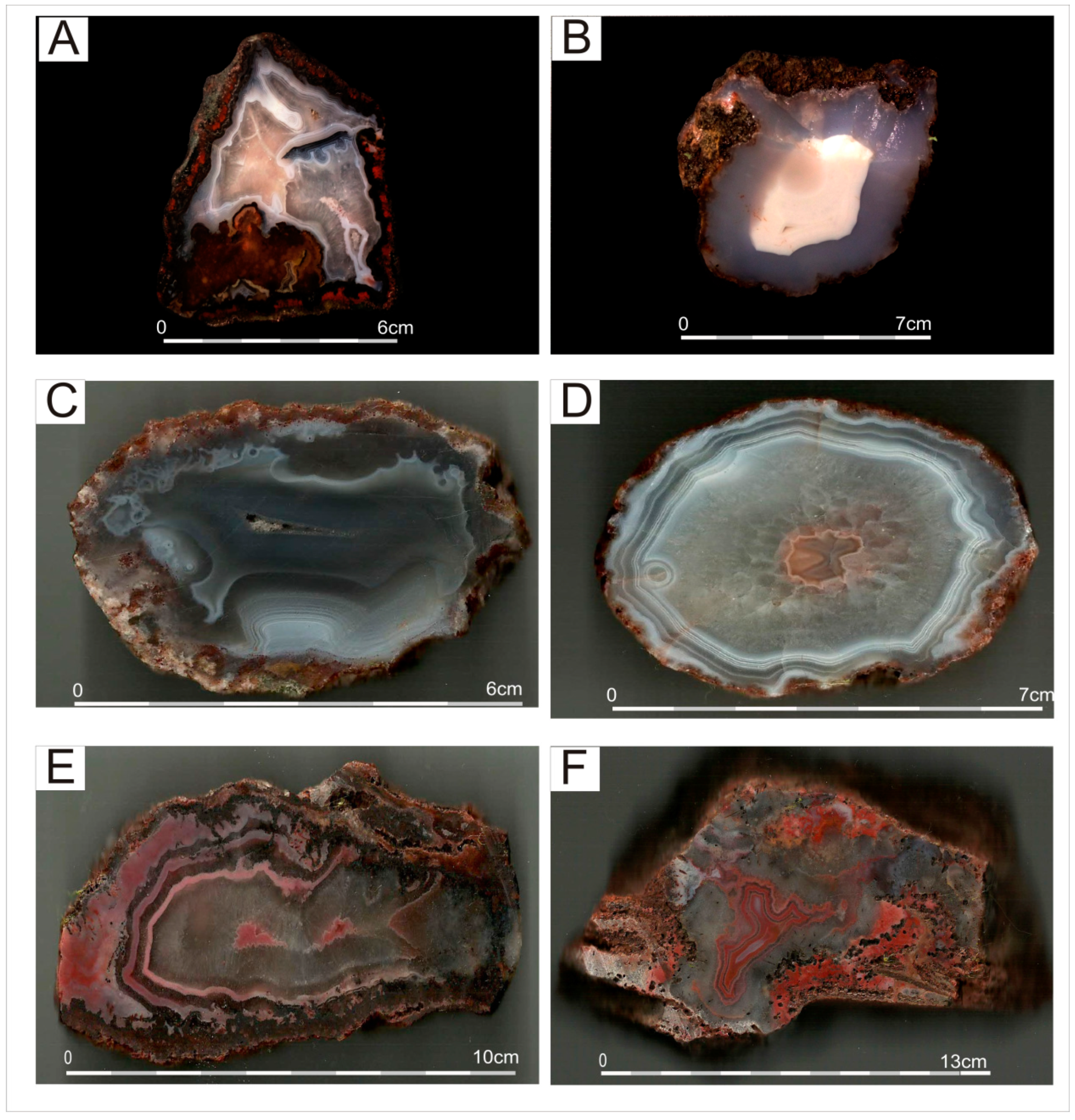
4.1. Field and Macroscopic Observations
4.2. Microscopic Characteristics in Transmitted and Reflected Light
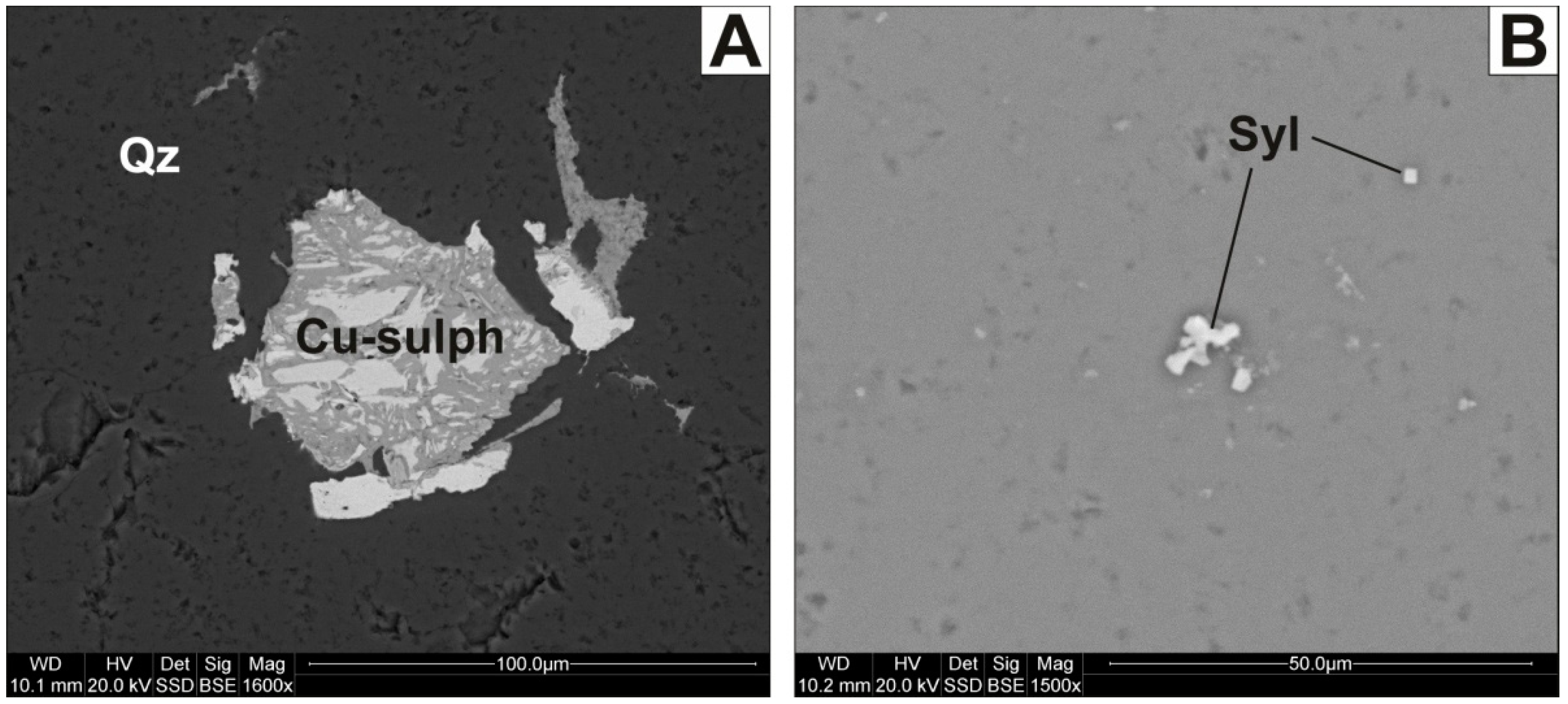
4.3. Scanning Electron Microscopy (SEM)
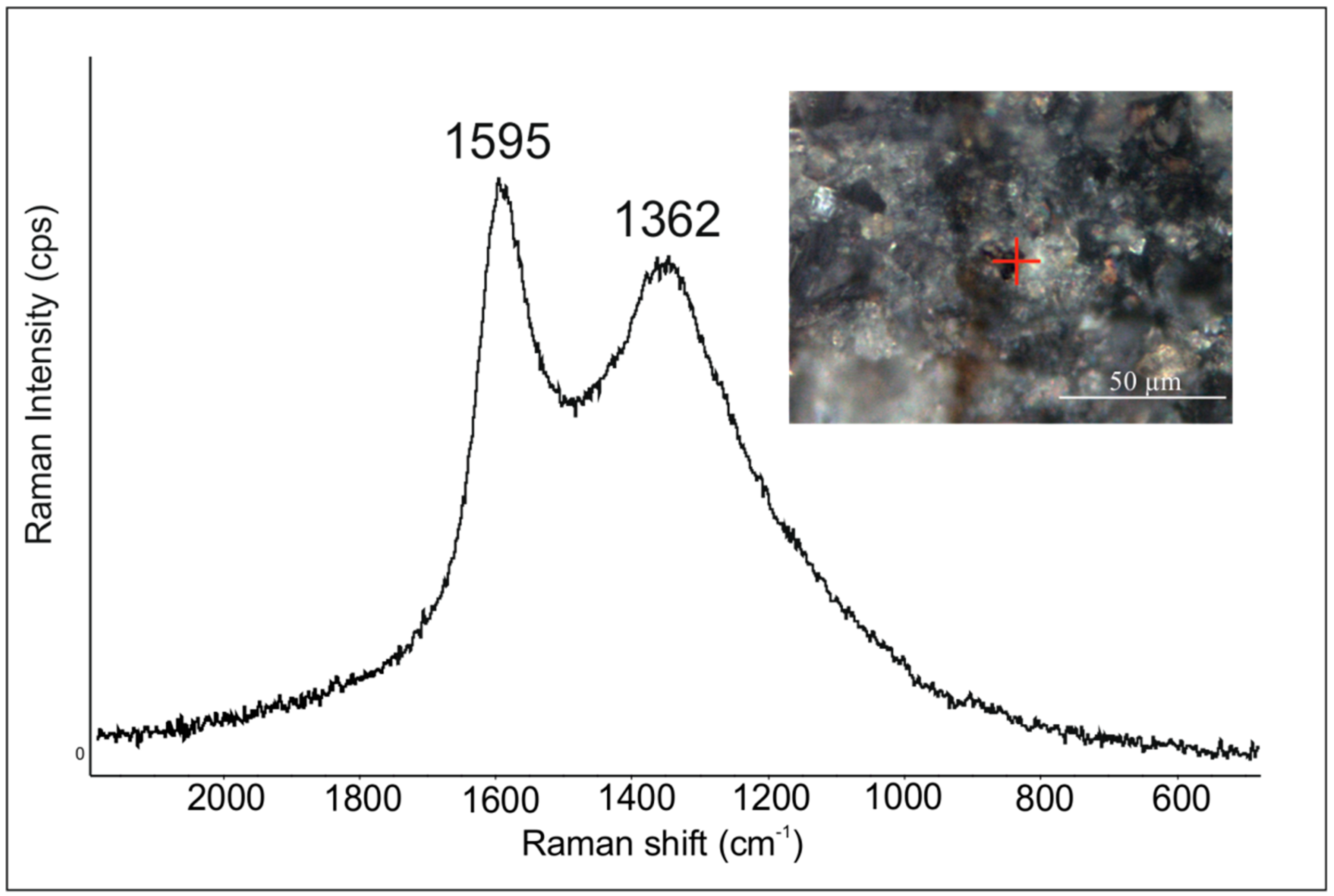
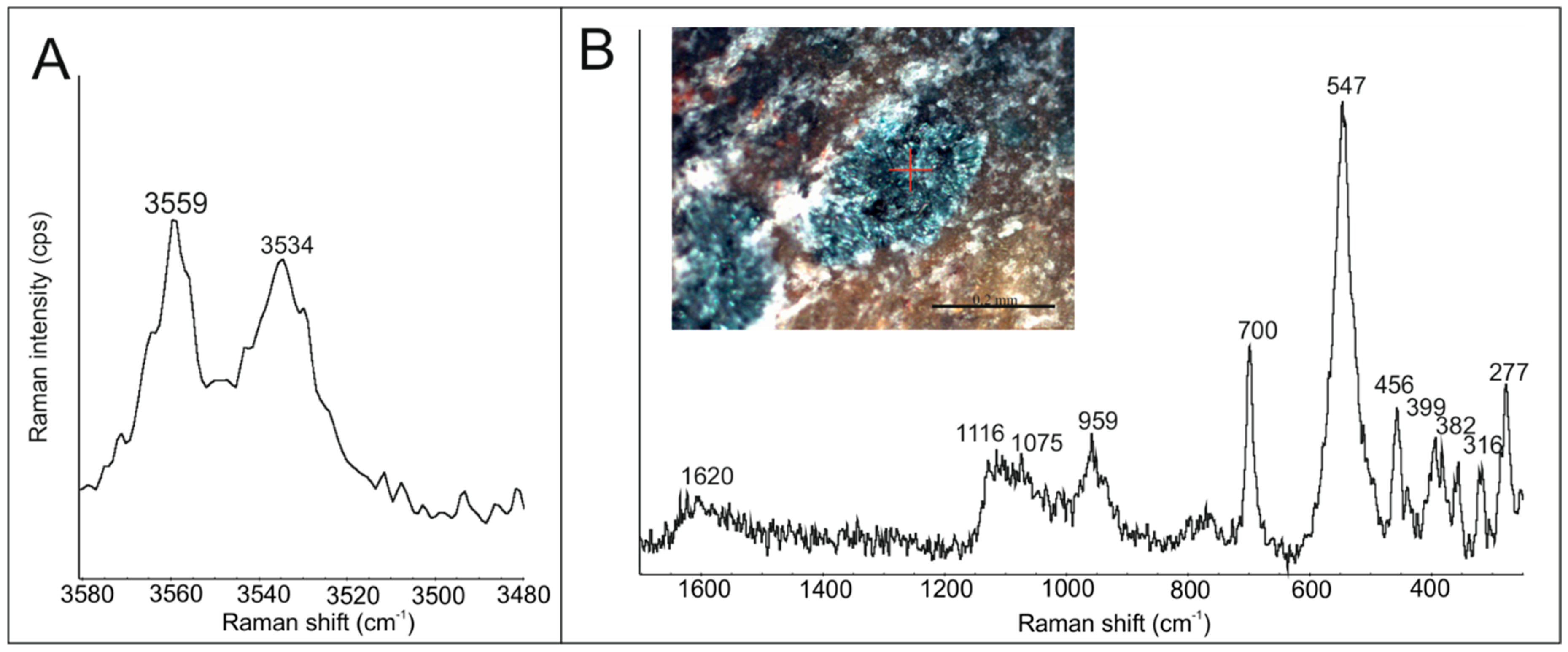
4.4. Raman Micro-Spectroscopy (RS)
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Götze, J.; Plötze, M.; Tichomirowa, M.; Fuchs, H.; Pilot, J. Aluminum in quartz as an indicator of the temperature of formation of agate. Mineral. Mag. 2001, 65, 407–413. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R.; Kempe, U.; Kapitonov, I.; Vennemann, T. Characteristics and origin of agates in sedimentary rocks from the Dryhead area, Montana, USA. Mineral. Mag. 2009, 73, 673–690. [Google Scholar] [CrossRef]
- Götze, J. Agate-Fascination between Legend and Science; Agates Zenz, J., III, Ed.; Bode-Verlag: Salzhemmendorf, Germany, 2011; pp. 19–133. [Google Scholar]
- Moxon, T.; Nelson, D.R.; Zhang, M. Agate recrystallization: Evidence from samples found in Archaean and Proterozoic host rocks, Western Australia. Aust. J. Earth Sci. 2006, 53, 235–248. [Google Scholar] [CrossRef]
- Heaney, P.J. A proposed mechanism for the growth of chalcedony. Contrib. Mineral. Petrol. 1993, 115, 66–74. [Google Scholar] [CrossRef]
- Graetsch, H. Structural characteristics of opaline and microcrystalline silica minerals. Rev. Mineral. Geochem. 1994, 29, 209–232. [Google Scholar]
- Götze, J.; Nasdala, L.; Kleeberg, R.; Wenzel, M. Occurrence and distribution of “moganite” in agate/chalcedony: A combined micro-Raman, Rietveld, and cathodoluminescence study. Contrib. Mineral. Petrol. 1998, 133, 96–105. [Google Scholar] [CrossRef]
- Moxon, T.; Ríos, S. Moganite and water content as a function of age in agate: An XRD and thermogravimetric study. Eur. J. Mineral. 2004, 16, 269–278. [Google Scholar] [CrossRef]
- Götze, J.; Nasdala, L.; Kempe, U.; Libowitzky, E.; Rericha, A.; Vennemann, T. The origin of black colouration in onyx agate from Mali. Miner. Mag. 2012, 76, 115–127. [Google Scholar] [CrossRef]
- Richter, S.; Götze, J.; Niemeyer, H.; Möckel, R. Mineralogical investigations of agates from Cordón de Lila, Chile. Andean Geol. 2015, 42, 386–396. [Google Scholar]
- French, M.W.; Worden, R.H.; Lee, D.R. Electron backscatter diffraction investigation of length-fast chalcedony in agate: Implications for agate genesis and growth mechanisms. Geofluids 2013, 13, 32–44. [Google Scholar] [CrossRef]
- Sobczak, N.; Sobczak, T. Big Encyclopedy of Gemstones; Polskie Wydawnictwa Naukowe Publishing House: Warsaw, Poland, 1998; p. 422. (In Polish) [Google Scholar]
- Manecki, A. Agates and Silicites: Genesis of Beauty-a Beauty of Genesis; AGH—The University of Science and Technology Publishing House: Krakow, Poland, 2015; p. 90. (In Polish) [Google Scholar]
- Żaba, J. Ilustrated Dictionary of Rocks and Minerals; Videograf, II: Katowice, Poland, 2003; p. 503. (In Polish) [Google Scholar]
- Jahn, S.; Bode, R.; Lyckberg, P.; Medenbach, O.; Lierl, H.-J. Marokko. Land der Schönen Mineralien und Fossilien; Bode-Verlag GmbH: Salzhemmendorf, Germany, 2003; p. 535. (In Germany) [Google Scholar]
- Zenz, J. Achate; Bode-Verlag GmbH: Salzhemmendorf, Germany, 2005; p. 656. (In Germany) [Google Scholar]
- Zenz, J. Achate-Schätze; Bode-Verlag GmbH: Salzhemmendorf, Germany, 2009; p. 656. (In Germany) [Google Scholar]
- Bakarat, A. Gold mineralization in the Ourika Gneiss (High Atlas of Marrakech, Morocco): Mineral paragenesis and fluid P-T-X evolution. Arabian J. Geosci. 2015, 8, 3207–3222. [Google Scholar] [CrossRef]
- Ilmen, S.; Alansari, A.; Bajddi, A.; Ennaciri, A.; Maacha, L. Contribution à l’étude géologique du gisement à Cu, Zn, Pb et Ag-Au d’Amensif (District minier d’Amizmiz, Haut Atlas occidental, Maroc). Int. J. Innov. Appl. Stud. 2014, 6, 768–783. (In French) [Google Scholar]
- Lafforgue, L.; Barbarand, J.; Missenard, Y.; Brigaud, B.; Saint-Bézar, B.; Yans, J.; Dekoninck, A.; Saddiqi, O. Origin of the Bouarfa Manganese Ore Deposit (Eastern High Atlas, Morocco): Insights from Petrography and Geochemistry of the Mineralization. Mineral resources in a sustainable world. In Proceedings of the 13th SGA Biennial Meeting 2015, Nancy, France, 24–27 August 2015; pp. 1949–1952. [Google Scholar]
- Rddad, L.; Bouhlel, S. The Bou Dahar Jurassic carbonate-hosted Pb–Zn–Ba deposits (Oriental High Atlas, Morocco): Fluid-inclusion and C–O–S–Pb isotope studies. Ore Geol. Rev. 2016, 72, 1072–1087. [Google Scholar] [CrossRef] [Green Version]
- Essalhi, M.; Mrani, D.; Essalhi, A.; Toummite, A.; Ali-Ammar, H. Evidence of a high quality barite in Drâa-Tafilalet region, Morocco: A nonupgraded potential. J. Mater. Environ. Sci. 2018, 9, 1366–1378. [Google Scholar]
- Verhaert, M.; Bernard, A.; Saddiqi, O.; Dekoninck, A.; Essalhi, M.; Yans, J. Mineralogy and genesis of the polymetallic and polyphased low grade Fe–Mn–Cu ore of Jbel Rhals deposit (Eastern High Atlas, Morocco). Minerals 2018, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Dumańska-Słowik, M.; Natkaniec-Nowak, L.; Wesełucha-Birczyńska, A.; Gaweł, A.; Lankosz, M.; Wróbel, P. Agates from Sidi Rahal, in the Atlas Mountains of Morocco: Gemological characteristics and proposed origin. Gems Gemol. 2013, 49, 148–159. [Google Scholar] [CrossRef]
- Natkaniec-Nowak, L.; Dumańska-Słowik, M.; Pršek, J.; Lankosz, M.; Wróbel, P.; Gaweł, A.; Kowalczyk, J.; Kocemba, J. Agates from Kerrouchen (the Atlas Mountains, Morocco): Textural Types and Their Gemmological Characteristics. Minerals 2016, 6, 77. [Google Scholar] [CrossRef]
- Ellero, A.; Ottria, G.; Malusà, M.G.; Ouanaimi, H. Structural Geological Analysis of the High Atlas (Morocco): Evidences of a Transpressional Fold-Thrust Belt, Chapter 9 in Sharkov E.; Tectonics-Recent Advances; InTech: Rijeka, Croatia, 2012; p. 323. [Google Scholar]
- Choubert, G.; Marais, J. Geologie du Marocco. In Proceedings of the 19th International Geological Congress, Algiers, Algeria, 8–15 September 1952. [Google Scholar]
- Laville, E. Role of the Atlas Mountains (northwest Africa) within the African-Eurasian plate-boundary zone: Comment. Geology 2002, 30, 1–95. [Google Scholar] [CrossRef]
- Lovering, T.G. Jasperoid in the United States; its Characteristics, Origin, and Economic Significance (No. 710); USGS: Reston, VA, USA, 1972; p. 176.
- Dong, G.; Morrison, G.; Jaireth, S. Quartz textures in epithermal veins, Queensland; classification, origin and implication. Econ. Geol. 1995, 90, 1841–1856. [Google Scholar] [CrossRef]
- Bambauer, H.U.; Brunner, G.O.; Laves, F. Beobachtungen über Lamellen bau an Bergkristallen. Z. Krist. 1961, 116, 173–181. (In German) [Google Scholar] [CrossRef]
- Moncada, D.; Mutchler, S.; Nieto, A.; Reynolds, T.J.; Rimstidt, J.D.; Bodnar, R.J. Mineral textures and fluid inclusion petrography of the epithermal Ag–Au deposits at Guanajuato, Mexico: Application to exploration. J. Geochem. Explor. 2012, 114, 20–35. [Google Scholar] [CrossRef]
- Legodi, M.; de Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigment. 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Hanesch, M. Raman spectroscopy of iron oxides and (oxy)hydroxides at low laser power and possible applications in environmental magnetic studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman spectrum of titanium dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Kharbish, S. Raman spectroscopic features of Al- Fe3+- poor magnesiochromite and Fe2+- Fe3+- rich ferrian chromite solid solutions. Mineral. Petrol. 2017, 112, 245–256. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffe, B.; Petitet, J.-P.; Froigneux, E.; Moreau, M.; Rouzaud, J.-N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta Part A 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- Starkey, N.A.; Franchi, I.A.; Alexander, C.M.O. A Raman spectroscopic study of organic matter in interplanetary dust particles and meteorites using multiple wavelength laser excitation. Meteorit. Planet. Sci. 2013, 48, 1800–1822. [Google Scholar] [CrossRef] [Green Version]
- Ospitali, F.; Bersani, D.; Di Lonardo, G.; Lottici, P. ‘Green earths’: Vibrational and elemental characterization of glauconites, celadonites and historical pigments. J. Raman Spectrosc. 2008, 39, 1066–1073. [Google Scholar] [CrossRef]
- Buzgar, N.; Apopei, A.I. The Raman study of certain carbonates. Geol. Tomul L 2009, 2, 97–112. [Google Scholar]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Dimova, M.; Panczer, G.; Gaft, M. Spectroscopic study of barite from the Kremikovtsi deposit (Bulgaria) with implication for its origin. Ann. Geol. Penins. Balk. 2006, 67, 101–108. [Google Scholar] [CrossRef]
- White, S.N. Laser Raman spectroscopy as a technique for identification of seafloor hydrothermal and cold seep minerals. Chem. Geol. 2009, 259, 240–252. [Google Scholar] [CrossRef]
- Marshall, C.P.; Marshall, A.O. Raman Hyperspectral Imaging of Microfossils: Potential Pitfalls. Astrobiology 2013, 13, 920–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouketsu, Y.; Mizukami, T.; Mori, H.; Endo, S.; Aoya, M.; Hara, H.; Nakamura, D.; Wallis, S. A new approach to develop the Raman carbonaceous material geothermometer for low-grade metamorphism using peak width. Isl. Arc 2014, 23, 33–50. [Google Scholar] [CrossRef]
- Aoya, M.; Kouketsu, Y.; Endo, S.; Shimizu, H.; Mizukami, T.; Nakamura, D.; Wallis, S. Extending the applicability of the Raman carbonaceous-material geothermometer using data from contact metamorphic rocks. J. Metamorph. Geol. 2010, 28, 895–914. [Google Scholar] [CrossRef]
- Constantina, C.; Moxon, T. Agates from Gurasada, Southern Apuseni Mountains, Romania: An XRD and Thermogravimetric study. Carpathian J. Earth Environ. Sci. 2010, 5, 89–99. [Google Scholar]
- Zhang, M.; Moxon, T. Infrared absorption spectroscopy of SiO2-moganite. Am. Mineral. 2014, 99, 671–680. [Google Scholar] [CrossRef]
- Folk, R.L.; Pittman, J.S. Length-slow chalcedony; a new testament for vanished evaporites. J. Sediment. Res. 1971, 41, 1045–1058. [Google Scholar]
- Keene, J.B. Chalcedonic quartz and occurrence of quartzine (length-slow chalcedony) in pelagic sediments. Sedimentology 1983, 30, 449–454. [Google Scholar] [CrossRef]
- Rykart, R. Quarz-Monographie; Ott Verlag: Thun, Switzerland, 1989; p. 462. (In German) [Google Scholar]
- Fournier, R.O. The behavior of silica in hydrothermal solutions. Rev. Econ. Geol. 1985, 2, 45–60. [Google Scholar]
- Saunders, J.A. Silica and gold textures in bonanza ores of the Sleeper Deposit, Humboldt County, Nevada; evidence for colloids and implications for epithermal ore-forming processes. Econ. Geol. 1994, 89, 628–638. [Google Scholar] [CrossRef]
- Yilmaz, T.I.; Duschl, F.; Di Genova, D. Feathery and network-like filamentous textures as indicators for the re-crystallization of quartz from a metastable silica precursor at the Rusey Fault Zone, Cornwall, UK. Solid Earth 2016, 6, 1509–1536. [Google Scholar] [CrossRef] [Green Version]
- Odin, G.S.; Desprairies, A.; Fullgar, P.D.; Bellon, H.; Decarreau, A.; Fröhlich, F.; Zelvelder, M. Nature and geological significance of celadonite. In Green Marine Clays: Oolitic Ironstone Facies, Verdine Facies, Glaukony Facies and Celadonite-Bearing Rock Facies-A Comparative Study; Odin, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 337–392. [Google Scholar]
- Baker, L.L.; Rember, W.C.; Sprenke, K.F.; Strawn, D.G. Celadonite in continental flood basalts of the Columbia River Basalt Group. Am. Mineral. 2012, 97, 1284–1290. [Google Scholar] [CrossRef]
- Kříbek, B.; Sýkorová, I.; Machovič, V.; Knésl, I.; Laufek, F.; Zachariáš, J. The origin and hydrothermal mobilization of carbonaceous matter associated with Paleoproterozoic orogenic-type gold deposits of West Africa. Precambrian Res. 2015, 270, 300–317. [Google Scholar] [CrossRef]
- Moxon, T.; Carpenter, M.A. Crystallite growth kinetics in nanocrystalline quartz (agate and chalcedony). Mineral. Mag. 2009, 73, 551–568. [Google Scholar] [CrossRef]
- Powolny, T.; Dumańska-Słowik, M.; Sikorska-Jaworowska, M.; Wójcik-Bania, M. Agate mineralization in spilitized Permian volcanics from “Borówno” quarry (Lower Silesia, Poland) –microtextural, mineralogical, and geochemical constraints. Ore Geol. Rev. 2019, 114, 103–130. [Google Scholar] [CrossRef]


| Raman Bands (cm−1) | Solid Phase | References |
|---|---|---|
| 463 (s), 357 (w), 210 (m), 130 (m) | low quartz | [7] |
| 502 (s) | moganite | [7] |
| 1323 (s), 669 (w), 613 (m), 497 (w), 413 (s), 293 (s), 248 (m), 226 (s) | hematite | [33,34] |
| 1316 (m), 685 (m), 553 (m), 388 (s), 301 (m), 247 (w) | goethite | [33] |
| 610 (s), 443 (s) | rutile | [35] |
| 708–688 (s), 569 (w) | magnesiochromite | [36] |
| 1595 (s), 1362 (s) | bituminous matter | [37,38] |
| 3559 (m), 3534 (m), 1620 (m), 1116 (w), 1075 (w), 959 (m), 700 (s), 547 (s), 456 (m), 399 (w), 382 (w), 277 (m) | celadonite | [39] |
| 1088 (s), 714 (w), 285 (m), 158 (m) | calcite | [40,41] |
| 1142 (w), 989 (s), 649 (w), 619 (w), 465 (m), 456 (w) | barite | [42,43] |
| Analytical Material | Raman Band Integral Ratio I(502)/I(463) | Local Moganite Content | ||||
|---|---|---|---|---|---|---|
| (%) | Mean (%) | Standard Deviation | (wt.%) | Mean (%) | Standard Deviation | |
| Asni (11) | 0.0–23.2 | 13.7 | 7.2 | 0.0–56.0 | 38.3 | 17.4 |
| Agouim (34) | 0.0–65.2 | 9.1 | 11.8 | 0.0–78.0 | 25.5 | 18.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pršek, J.; Dumańska-Słowik, M.; Powolny, T.; Natkaniec-Nowak, L.; Toboła, T.; Zych, D.; Skrepnicka, D. Agates from Western Atlas (Morocco)—Constraints from Mineralogical and Microtextural Characteristics. Minerals 2020, 10, 198. https://doi.org/10.3390/min10020198
Pršek J, Dumańska-Słowik M, Powolny T, Natkaniec-Nowak L, Toboła T, Zych D, Skrepnicka D. Agates from Western Atlas (Morocco)—Constraints from Mineralogical and Microtextural Characteristics. Minerals. 2020; 10(2):198. https://doi.org/10.3390/min10020198
Chicago/Turabian StylePršek, Jaroslav, Magdalena Dumańska-Słowik, Tomasz Powolny, Lucyna Natkaniec-Nowak, Tomasz Toboła, Damian Zych, and Dominika Skrepnicka. 2020. "Agates from Western Atlas (Morocco)—Constraints from Mineralogical and Microtextural Characteristics" Minerals 10, no. 2: 198. https://doi.org/10.3390/min10020198





